Point of Care Molecular Diagnostics Market by
Product & Service (Assays, Kits, Analyzers, Software), Application
(Respiratory Disease, Hospital Acquired Infection), Technology (RT-PCR, INAAT),
End User (Physician Office, ICUS) - Global Forecast to 2023", The global point
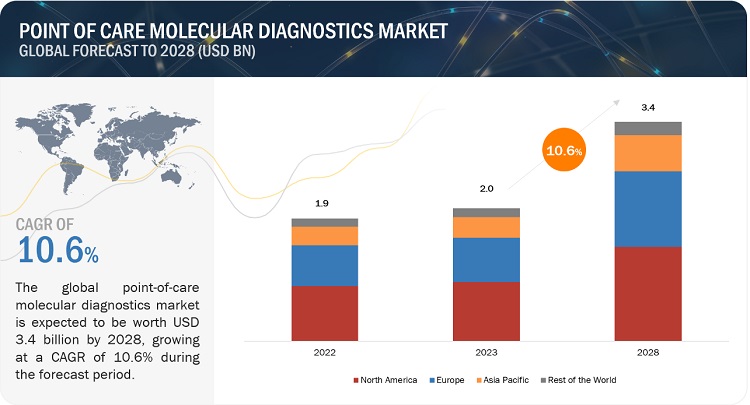
of care (POC) molecular diagnostics market is expected to reach USD 1440.2
Million by 2023 from USD 725.5 Million in 2018, at a CAGR of 14.7%.
Download FREE Brochure @ https://www.marketsandmarkets.com/pdfdownload.asp?id=143524127
Detailed analysis of POC molecular diagnostics market in Asia Pacific countries such as China, Japan, India, and others
Download FREE Brochure @ https://www.marketsandmarkets.com/pdfdownload.asp?id=143524127
Growth in the point-of-care (POC) molecular diagnostics market is mainly
driven by factors such as the increasing worldwide prevalence of infectious
diseases, rising focus on decentralized diagnostics, and the growing demand for
CLIA-waived molecular POC tests.
Product Analysis
Product
matrix, which gives a detailed comparison of the product portfolios of each
company
Company Information
Detailed
analysis and profiling of additional market players (up to 5)
Geographic Analysis
Detailed
analysis of POC molecular diagnostics market in European countries such as the
UK, Germany, France, Italy, Spain, and others
Detailed analysis of POC molecular diagnostics market in Asia Pacific countries such as China, Japan, India, and others
Growing demand for CLIA-waived respiratory test
to drive the global point-of-care molecular diagnostics market close to USD
1.44 billion by 2023
Clinical
Laboratory Improvement Amendments (CLIA), passed in 1988, aimed to establish
quality standards for all laboratory testing procedures to ensure the accuracy,
reliability, and timeliness of patient test results regardless of where the
test was performed. As defined by CLIA, waived tests are categorized as “simple
laboratory examinations and procedures that have an insignificant risk of an
erroneous result.” The Food and Drug Administration (FDA) determines the
criteria for tests being simple with a low risk of error and approves
manufacturer’s applications for test system waiver. In the past 2 years, the
POC molecular diagnostics industry has witnessed a high number of CLIA-waived
tests.
Some of the key CLIA waivers granted are as follows:
- In 2017, Alere Inc. (US)
received a CLIA waiver from the FDA for the Alere i RSV Rapid Molecular
Test used to detect RSV (respiratory syncytial virus) infections.
- In 2016, Roche Diagnostics
Limited (Switzerland) was granted a CLIA waiver from the FDA for the cobas
Influenza A/B & RSV test to be used on its cobas Liat System. This was
the first CLIA-waived PCR test used to detect Influenza A/B & RSV
within 20 minutes.
Increasing prevalence of diseases such as
Influenza A/B,RSV, Hospital Acquired Infections (HAIs) likely to increase the
demand for POC molecular tests
Read More:
https://www.marketsandmarkets.com/Market-Reports/point-of-care-molecular-diagnostic-market-143524127.html
Read More:
https://www.marketsandmarkets.com/Market-Reports/point-of-care-molecular-diagnostic-market-143524127.html



No comments:
Post a Comment